December 2025 - Dr. Steven Horwitz (Memorial Sloan Kettering Cancer Center) provides an introduction to ONO-4685 and its clinical trial. Dr. Horwitz describes how this potential treatment for T-cell lymphomas works. He also discusses the Phase 1 clinical trial process, who might be good candidates to participate, and what they should consider when making a decision to join a clinical trial.
Dr. Steven Horwitz is a medical oncologist specializing in lymphoma care and research. He focuses on advancing therapies for rare lymphomas, including T-cell lymphomas, cutaneous lymphomas, Hodgkin disease, and post-transplant lymphoproliferative disorders (PTLD). He serves as Principal Investigator for the ONO-4685 clinical trial at Memorial Sloan Kettering.
April 2025 - ONO Pharmaceuticals abstract entitled "ONO-4685: A Novel PD-1/CD3 Bispecific T-Cell Engager (TCE) for the Treatment of T-Cell Malignancies" was included in the 2025 Annual Meeting Supplement for the American Association for Cancer Research.
Click on the thumbnail below to see an enlarged view of the abstract of this potential new treatment for cutaneous lymphoma.
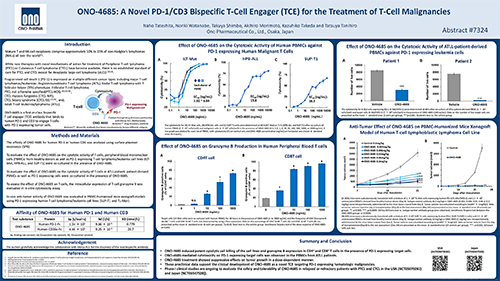
Naho Tateshita, Noriki Watanabe, Takuya Shimbo, Akihiro Morimoto, Kazuhiko Takeda, Tatsuya Tanihiro. ONO-4685: A novel PD-1/CD3 bispecific T-cell engager (TCE) for the treatment of T-cell malignancies [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2025; Part 1 (Regular Abstracts); 2025 Apr 25-30; Chicago, IL. Philadelphia (PA): AACR; Cancer Res 2025;85(8_Suppl_1):Abstract nr 7324.
Clinical Trial Title
Study of ONO-4685 in Patients With Relapsed or Refractory T Cell Lymphoma
Clinical Trial Description
The primary objective of this Phase I study is to evaluate the safety, tolerability, pharmacokinetics, and preliminary efficacy of ONO-4685 given as monotherapy in patients with relapsed or refractory Peripheral T-Cell Lymphoma (PTCL) and Cutaneous T-Cell Lymphoma (CTCL). ONO-4685 is a bispecific antibody that can bind to two target proteins: PD-1 on cancer cells and CD3 on T-cells which may induce the anti-cancer activity of T-cells. Study participants will receive ONO-4685 by intravenous (IV) infusion. Response to the study drug will be measured at regular timepoints throughout the study using standard PTCL and CTCL assessment tools.
